
Smarter science.
Better health.

JULY 2017
RDD Pharma, a leader in developing treatments for anorectal disorders, has announced that the European Medicines Agency’s Committee for Orphan Medicinal Products has granted orphan designation for RDD-0315, an investigational drug for the treatment of fecal incontinence in patients with spinal cord injury. The committee recognised taht RDD-0315 may be of significant benefit to spinal cord injury patients affected by fecal incontinence.
Orphan Designation provides potential incentives from the EU such as: protocol assistance; reduced fees; funding for clinical trials; and protection from competition once the medicine is placed on the market, including 10 years of market exclusivity.
Positive Phas 2a results in evaluating the safety and efficacy of RDD-0315 have been reported with a statistically significant reduction in the number of fecal incontinence episodes at 8 and 12 hours post-administration.
An estimated 20 million adults suffer from fecal incontinence in the U.S.
About RDD-1219 and Chronic Anal Fissure: RDD-1219 Capository™ has the potential to provide pain relief and to promote healing of the extremely painful and difficult to treat condition of chronic anal fissure. There are an estimated 235,000 new cases of anal fissure reported every year in the US, and about 40% of them persist for months and even years. M.H. Madalinski. World J Gastrointestinal Pharmacology Ther 2011 April 6; 2(2): 9-16
The Pipeline
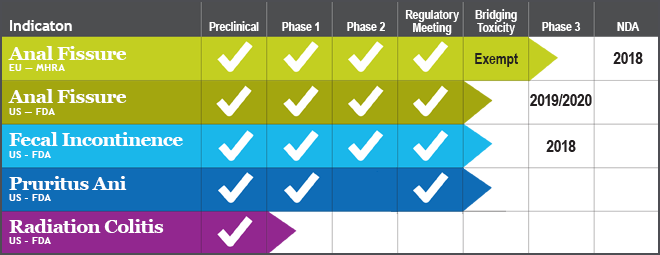
RDD is initiating a Phase 3 study for treatment of chronic anal fissure with RDD 1219, with a novel sustained release drug-delivery device — the Capository™. This product has numerous competitive advantages over the one marketed product for this indication. The anal fissure market is anticipated to reach $5-600M.
RDD recently completed a successful Phase 2a study of RDD 0315 in fecal incontinence, which reached the primary endpoint (lowered frequency of incontinence events). There are no approved therapies for this indication. Fecal incontinence market has the potential to approach $3B in sales.


